
|

|
No. 84 October 2012 |
| |
Bioscience
Better Approach to Cancer Diagnosis
Prof. Yan Xiyun¡¯s group at the Institute of Biophysics, CAS, in collaboration with Prof. Pan Yongxin¡¯s group at the Institute of Geology and Geophysics, CAS, biomimetically synthesized a new magnetoferritin nanoparticle for cancer diagnosis and treatment. The new nanoparticle has an iron oxide core encapsulated in a ferritin protein shell. The protein shell binds specifically to tumor cells, while the inorganic core exhibits peroxidase activity that visualizes the tumors in the presence of chromogen substrates. The examination of 474 clinical specimens from patients with nine types of cancer verified that these nanoparticles can distinguish cancerous cells from normal cells with a sensitivity of 98% and specificity of 95%. Compared with traditional antibody-based immunohistochemistry, this new magnetoferritin nanoparticle-based method is more rapid (It only takes 1 hour, rather than 4 hours in immunohistochemistry) and simpler to implement since it achieves tumors targeting and visualization in one step, avoiding multi-step incubations with expensive and unstable antibodies, and repeated washing procedures of immunohistochemistry. This greatly shortens diagnostic time and reduces the cost, and thus has significant implications for cancer diagnosis. This work provides new ideas, new reagents and new technologies for cancer diagnosis and treatment, of which the result published in Nature Nanotechnology online on June 17, 2012.
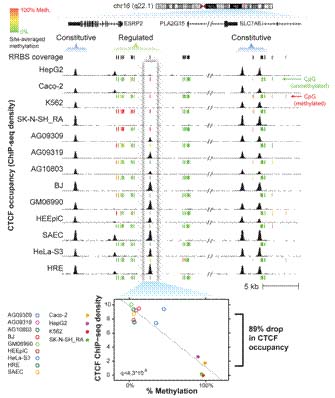
Widespread Plasticity in CTCF Occupancy Linked to DNA Methylation
Recently, Dr. Qu Hongzhu from the research group headed by Prof. Fang Xiangdong at the Lab of Disease Genomics and Individualized Medicine, Beijing Institute of Genomics, CAS, and her collaborators from the lab leaded by Dr. John A. Stamatoyannopoulis, one of the ENCODE project leaders and director of the Northwest Reference Epigenome Mapping Center, NIH, found a tight linkage between DNA methylation and the global occupancy patterns of CTCF, a major sequence-specific multiple functional regulatory factor. They analyzed genome-wide occupancy patterns of CTCF by ChIP-seq in 19 diverse human cell types, including normal primary cells and immortal lines and observed that 64% of CTCF sites vary in at least one cell type, demonstrating the existence of a widespread variability in CTCF occupancy. Comparison with massively parallel bisulfite sequencing data, they observed that 41% of variable CTCF binding is linked to differential DNA methylation, concentrated at two critical positions within the CTCF recognition sequence. CTCF binding patterns were markedly different in normal versus immortal cells, with the latter showing widespread disruption of CTCF binding associated with increased methylation. The research not only enriched the scientific theory of eukaryotic gene expression and regulation, but also obtained new epigenetic targets affecting tissue differentiation and tumorigenesis. The paper titled "widespread plasticity in CTCF occupancy linked to DNA methylation" was published in Genome Research (2012; 22:1680¨C8).
New Approach to Cancer Diagnosis and Therapy
A research article, titled ¡°tuning the autophagy-inducing activity of lanthanide-based nanocrystals through specific-surface coating peptides¡±, was published in a recent issue of Nature Materials. The research team, led by Prof. Wen Longping at the School of Life Science, University of Science & Technology of China, employed a series of small surface-coating peptides to modulate the autophagy-inducing activity of nanomaterials, thus achieving reduced toxicity and enhanced tumor cell killing for the nanomaterials. The work may provide a new approach to enable the applications of nanomaterials in cancer diagnosis and therapy. Using phage display, the researchers discovered a short peptide RE-1, which binds to rare earth oxide and rare earth upconversionnanocrystals with high specificity. RE-1 forms a stable coating layer on the surface of nanocrystals, prevents nanocrystal sedimentation, and reduces nanocrystal-cell interaction. As a result, the autophagy-inducing activity of the nanocrystals is effectively abrogated, leading to reduced toxicity and improved bio-safety for the nanomaterials. On the other hand, the addition of an arginine-glycine-aspartic-acid motif to RE-1 enhances autophagy for the nanocrystals in cancer cells that over-expressintegrins, resulting in enhanced cytotoxicity. Thus, RE-1 and its variants provide a versatile tool for tuning material-cell interactions to achieve the desired level of autophagy, and may prove useful for the various diagnostic and therapeutic applications of lanthanide-based nanomaterials and nanodevices. The first author of the paper is Ms. Zhang Yunjiao, a Ph.D. candidate of the lab.
copyright © 1998-2015
CAS Newsletter Editorial Board: 52, Sanlihe Road, Beijing 100864,
CHINA
Email: slmi@cashq.ac.cn