
|

|
No. 85 December 2012 |
| |
Bioscience
Fully Human mAbs against Influenza A Virus Obtained
The 2009 pandemic H1N1 influenza virus caused huge loss to human society and became the main epidemic strain in recent years. Under the supervision of Prof. Sun Bing from the Molecular Virology Unit, Institut Pasteur of Shanghai, Hu Weibin and Chen Aizhong, isolated neutralizing antibodies against this virus. They separated pandemic H1N1 HA-specific memory B cells from a 2009 pandemic H1N1 influenza vaccine recipient with three surface markers: CD19, IgG, and HA-specific BCR, and identified the antibody variable genes of these memory B cells with single-cell RT-PCR. A panel of fully human monoclonal antibodies (mAbs) was obtained. The data showed that seven mAbs exhibited neutralizing activity against influenza A viruses of H1, H3, H5, H7 and H9 subtypes. Epitope mapping revealed that the seven mAbs targeted the HA2 fragment of the HA stem region, including a linear epitope locates on the fusion peptide and other conformational epitopes. Mechanism study indicated that these antibodies could inhibit the membrane fusion mediated by HA2 during virus entry. This finding may be helpful in the development of therapeutic antibodies and the design of universal vaccines for influenza A virus. This work was a collaboration between the Institute Pasteur of Shanghai and Antibody Research Center, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences. The research article entitled "Fully human broadly neutralizing monoclonal antibodies against influenza A viruses generated from the memory B cells of a 2009 pandemic H1N1 influenza vaccine recipient" was published on Virology on Oct. 16, 2012.
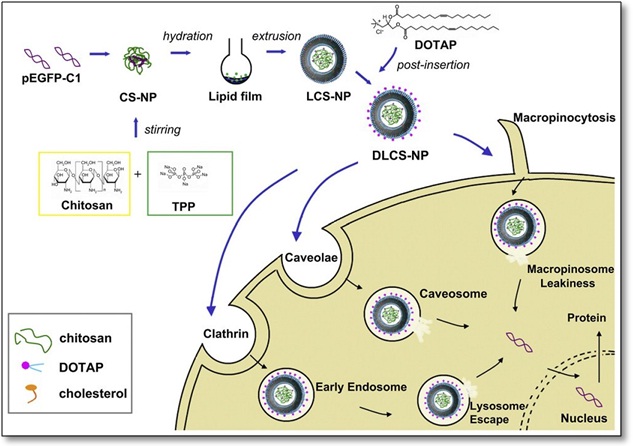
Progress Made in Ocular Gene Drug Delivery
Ocular diseases are usually treated with a topical application of drug solutions (i.e. eye drops). These traditional dosage forms account for nearly 90% of currently available marketed formulations due to their simplicity, safety and acceptance by patients. However, ocular physiological barriers and the poor water solubility of drug candidates present a number of problems for the development of ocular drug delivery systems. Recently, various ophthalmic drug delivery strategies aim at improving the bioavailability of poor soluble ocular drugs have emerged. New technological advances and developments of ocular drug delivery have been reviewed by Dr. Gan Yong¡¯s group from the Shanghai Institute of Materia Medica. Among them, lipid-based nanocarriers exhibit a variety of potential advantages as delivery systems for ocular administration. A novel ocular gene drug delivery system of cationic core-shell liponanoparticles (DLCS-NP) has been developed by Dr. Gan Yong¡¯s group. Researchers (Jiang Ming and Gan Li, et al) designed the DLCS-NP by the hydration of a thin lipid ?lm with a chitosan nanoparticle (CS-NP) suspension, followed by the post-insertion of the cationic phospholipid DOTAP (1,2-dioleoyl-3- trimethylammonium-propane). This specific delivery strategy has multiple functions, including better DNA protecting effect, good cellular uptake efficiency with multiple endocytic pathways, and endolysosome escaping ability. This research has been published on Biomaterials (2012, 33: 7621-7630).
copyright © 1998-2015
CAS Newsletter Editorial Board: 52, Sanlihe Road, Beijing 100864,
CHINA
Email: slmi@cashq.ac.cn