

A research team led by Professor Wu Haichen from the Institute of Chemistry of the Chinese Academy of Sciences (CAS), and Professor Liu Lei from Xihua University developed a nanopore-based enzyme-linked immunosorbent assay (NELISA) for cancer biomarker detection.
Early diagnosis of cancer is crucial for improving patient prognosis and significantly reducing mortality rates. The detection of cancer biomarkers in body fluids — being less harmful to the body — is more suitable for early-stage diagnosis. Among various methods, enzyme-linked immunosorbent assay (ELISA) is the most widely used and currently regarded as the gold standard for body fluid biomarker detection.
However, traditional ELISA relies on colorimetric signal output, which limits its sensitivity and multiplexing capability, making it insufficient for ultra-early cancer detection.
Although recent technological advancements have somewhat improved the performance of conventional ELISA, it still falls short of meeting the stringent demands of early-stage disease diagnosis.
The researchers employed a peptide probe, FGXD8⊂CB[7], containing various enzyme-responsive groups, as an enzymatic substrate in place of conventional chromogenic substrates. Following immune complex formation, an enzymatic reaction was initiated.
In the presence of the enzyme, the responsive groups dissociated, inducing structural changes in the probe. Consequently, the probe generated distinct translocation signals when passing through the wild-type α-hemolysin nanopore before and after enzymatic cleavage.
By statistically analyzing the frequency of current signals produced by FGXD8⊂CB[7], the researchers achieved quantitative detection of biomarker concentrations in the samples. Owing to the high signal specificity and stability of the peptide probes, the method enabled simultaneous detection of six biomarkers.
Finally, the researchers applied this approach to detect AFP, CEA, and CA19-9 in over 100 clinical blood samples, achieving a concordance rate exceeding 90 percent with standard clinical tests, thereby demonstrating the method’s strong potential for clinical applications.
This study integrates the strengths of ELISA and nanopore sensing technologies to create a highly sensitive, low detection limit (3–4 orders of magnitude lower than conventional methods), and multiplexed assay with strong anti-interference capability. It is expected to provide a powerful new tool for early cancer screening based on body fluid biomarker detection.
This study was published in Nature Nanotechnology.
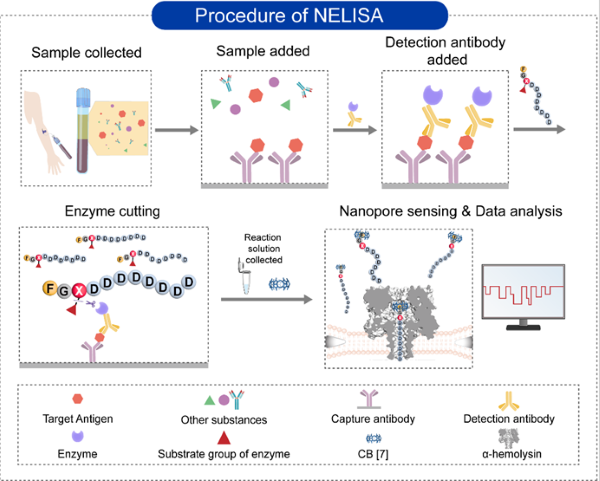
The design of peptide probes and procedure for NELISA
For more information, please contact:
Professor Wu Haichen
E-mail: haichenwu@iccas.ac.cn
Institute of Chemistry,
Chinese Academy of Sciences
Source: Institute of Chemistry,
Chinese Academy of Sciences