

Metabolic vulnerabilities driven by FGFR and EGFR gene alterations in cancer. [Image: Jin Nan]
Deregulated metabolism is a hallmark of cancer. It is believed to present new therapeutic opportunities and to attract increasing efforts in anticancer drug discovery. However, the metabolic vulnerabilities for most human cancers remain unclear.
In an article published in Nature Communications, a joint team from the Shanghai Institute of Materia Medica (SIMM), CAS and Xiamen University made progress in the identification of the metabolic vulnerabilities of receptor tyrosine kinases (RTK) aberrant cancer, the well-defined molecular subtypes in clinical cancer treatment.
The finding provides the possibility of tailoring metabolic inhibitors using known oncogenic alterations for cancer therapy. So far, very limited benefits have been obtained in the clinical modalities of metabolic targets, in which metabolic inhibitors were often delivered to broad cancer patients without indication of metabolic dependency.
With the advancement of metabolic inhibitors discovery, it is imperative to understand the patient stratification strategy for the treatment.
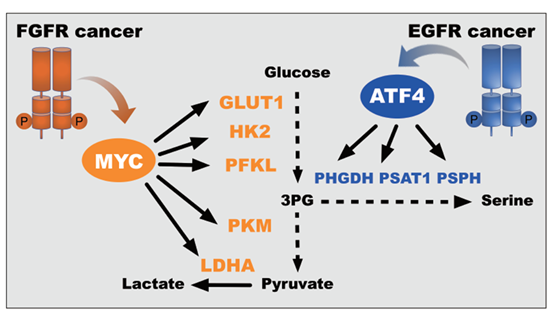
To stratify the responsive tumors to metabolism inhibitors, Huang Min, Geng Meiyu at the SMM and Lin Shuhai from Xiamen University took an approach to establish the linkage between metabolic dependency and the oncogenic alterations of receptor tyrosine kinases (RTK), the well-defined cancer genotypes occurred in a broad spectrum of cancer tissue types.
By integrating metabolomics and transcriptomics, they discovered that oncogenic RTK activation causes distinct metabolic preference. Specifically, epidermal growth factor receptor (EGFR) activation branches glucose metabolism to the serine synthesis for nucleotide biosynthesis and redox homeostasis, whereas fibroblast growth factor receptor (FGFR) activation recycles lactate to fuel mitochondrial phosphorylation for energy generation. Genetic alterations of EGFR and FGFR stratify the responsive tumors to pharmacological inhibitors that shut down the serine synthesis and lactate fluxes, respectively.
These findings provide a basis for stratifying EGFR and FGFR aberrant patients for metabolism-targeted therapies, suggesting a potential for precise and direct metabolic intervention to a broad patient population.
Linkage: https://www.nature.com/articles/s41467-019-10427-2
For more information, please contact:
Prof. Huang Min
Shanghai Institute of Materia Medica
Email: mhuang@simm.ac.cn
Source: Shanghai Institute of Materia Medica,
Chinese Academy of Sciences