

On Oct 25, 2018, a Tool Resource article contributed by Dr. Li Dong of the Institute of Biophysics of the Chinese Academy of Sciences (IBP/CAS), entitled "Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales" was published by Cell. This work developed the Grazing Incidence Structured Illumination Microscopy (GI-SIM), which is capable of high-speed, long-term, super-resolution imaging of dynamic intracellular processes. GI-SIM combines 97 nm resolution at a frame rate of 266 Hz with low rates of photo bleaching and phototoxicity to permit continuous imaging for hundreds to thousands of frames. Compared to our previously developed TIRF-SIM (Li Dong, et al., Science, 2015), GI-SIM probes ~10× further beyond the basal cell membrane and collects ~10× greater fluorescence signal. Compared with spinning disk confocal microscopy, GI-SIM achieves ~2× better spatial resolution and ~10× faster imaging speed. Compared with other super-resolution methods, GI-SIM achieves sub-100 nm resolution over a cellular-sized field-of-view ~100x faster over ~10-100-fold as many frames. In brief, GI-SIM achieves optimal 2D super-resolution imaging of multiple organelle dynamics within cells, which enables uncovering of a variety of organelle-organelle, organelle-cytoskeleton interactions.
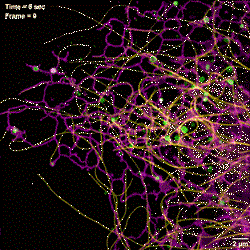
1. The researchers identified three novel mechanisms for generating ER tubules: hitchhiking, dTAC, and de novo budding.
2. They found that some moving late endosomes/lysosomes can bend, and even break, ER tubules. This observation provides a potential mechanism for how the ER can undergo fission, a process that has been minimally investigated.
3. They characterized the association of mitochondrial fusion with ER-mitochondrion contacts, and found that these contacts facilitate the coalescence of mitochondrial membranes.
4. They found hitchhiking interactions commonly occur among different organelles (i.e., one organelle contacts a different motile organelle and is pulled with it), and that this process can remodel both ER and mitochondrial networks.
5. They observed that the tubular ER undergoes ultra-dynamic segregation of its luminal sub domains.
6. They measured the heterogeneity of microtubule dynamic instability at unprecedented spatiotemporal resolution.
7. They found that ER-lysosome contacts play an important role in regulating lysosome positioning and distribution.
Dr. Li Dong and collaborators Dr. Eric Betzig and Dr. Jennifer Lippincott-Schwarts from the Janelia Research Campus, Howard Hughes Medical Institute, are co-corresponding authors. GuoYuting and Dr. Li Di from Dr. Li Dong’s group are the co-first authors, and Zhang Siwei from Dr. Li Dong’s group is the second author. This work was supported by the National Natural Science Foundation of China (NSFC), the Chinese Ministry of Science and Technology (MOST), the Chinese Academy of Sciences (CAS), and the Howard Hughes Medical Institute (HHMI).
For more information, please contact:
Li Dong
Institute of Biophysics, Chinese Academy of Sciences
Email: lidong@ibp.ac.cn