

The discovery of induced pluripotent stem cells (iPSCs) marked a milestone in the development of strategies in regenerative medicine (Takahashi and Yamanaka, 2006). However, the generation of iPSCs is a lengthy and inefficient procedure that requires many processes such as global remodeling of chromatin and resetting of the epigenome (Apostolou and Hochedlinger, 2013, Papp and Plath, 2013,Watanabe et al., 2013). In recent years, many efforts have been focused on the identification of important players that could either facilitate (Theunissen and Jaenisch, 2014) or hinder (Winzi et al., 2014) the reprogramming process, leading to the discovery of NANOG as one of the reprogramming facilitators (Silva et al., 2006, Silva et al., 2009). Although NANOG accelerates the induction of pluripotency, its mechanisms of action are only partially understood (reviewed in Saunders et al., 2013).
Recently, professor Faiola from the Research Center for Eco-Environment Sciences, CAS and Professor Wang from the Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA joined hands in collaboration on somatic cell reprogramming with a breakthrough in its molecular mechanisms, and discovered that the transcription factor NAC1 is involved in reprogramming somatic cells. This achievement was reported online in Stem Cell Reports on August 3, 2017.
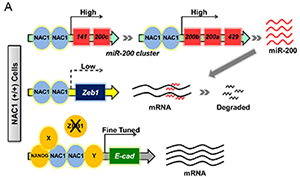
In their pursuit to identify pluripotency and reprogramming factors that may modulate NANOG functions in reprogramming, they examined additional components of the NANOG interactome (Costa et al., 2013, Wang et al., 2006). In particular they identified nucleus accumbens-associated protein 1 (NAC1), a stem cell-enriched factor that also interacts with OCT4 (Ding et al., 2012) and SOX2 (Ding et al., 2015). NAC1 belongs to the bric-a-brac tramtrac broad complex/pox virus and zinc-finger (BTB/POZ) family of transcription factors (Mackler et al., 2003), and is a ubiquitously expressed protein originally identified in the nucleus accumbens of the rat brain as a cocaine-inducible gene (Cha et al., 1997). Subsequently, NAC1 has been shown to play a role in the behavioral responses to psychostimulants (Mackler et al., 2000). In embryonic stem cells (ESCs), NAC1 is a common interacting partner (Wang et al., 2006) of, and upstream modulator (Kim et al., 2008) for, many pluripotency factors and epigenetic regulators. However, its mechanistic actions in pluripotency have not been defined. Besides being upregulated in pluripotent cells, NAC1 overexpression is also a hallmark of several types of cancers, including ovarian, cervical, and uterine (Ishikawa et al., 2010, Shih et al., 2011, Yeasmin et al., 2012). At the molecular level, NAC1 possesses a POZ domain N-terminally, and a BEN domain at the C terminus. The NAC1 POZ domain interacts with many factors, but is unique in that it does not contain a zinc-finger DNA-binding domain such as other POZ transcription factors. Therefore, it is believed that the NAC1 C-terminal BEN domain can mediate its binding to chromatin similarly to other BEN-containing transcriptional repressors (Dai et al., 2013).
Drs. Faiola and Wang have begun to investigate the role of NAC1 in the maintenance and establishment of pluripotency and demonstrated that Nac1 was surprisingly dispensable for early embryo development (Yap et al., 2013). Not unexpectedly, thereafter they were able to derive Nac1 knockout (KO) mouse embryonic stem cells (mESCs), which undergo normal self-renewal and maintain pluripotency (their unpublished data). In their study, they dissected the functional contribution of NAC1 in establishing pluripotency during somatic cell reprogramming. They identified a critical role for NAC1 in transcriptionally and post-transcriptionally modulating E-cadherin and Zeb1 expression during the generation of iPSCs. In the absence of NAC1 functions, reprogramming is diverted to an anomalous state that can be fully rescued with the re-expression of E-CADHERIN, but not NANOG or ESRRB. Their data thus uncover a previously unappreciated reprogramming factor that plays an indispensable role, beyond the mesenchymal-to-epithelial transition (MET), in controlling E-cadherin expression and establishing the bonafide pluripotency of iPSCs.
For more information, please contact:
Francesco Faiola
E-mail: faiola@rcees.ac.cn
or Yin Nuoya
E-mail address: nyyin@rcees.ac.cn
Research Center for Eco-Environmental Sciences, CAS
Source: Research Center for Eco-Environmental Sciences, CAS