

A team of scientists from the Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences (CAS), has determined the high-resolution atomic structure of a full-length class B G protein-coupled receptor (GPCR) that plays a key role in glucose homeostasis. This structure reveals, for the first time, the structural framework of different domains of a class B GPCR at high resolution and unexpectedly discloses many exciting molecular features, greatly deepening the understanding of signaling mechanisms of class B GPCRs.
In an article published online in Nature on May 17, 2017 (6:00 pm, London time) titled “Structure of the full-length glucagon class B G protein-coupled receptor”, scientists at SIMM, in collaboration with several groups based in China (Shanghai Tech University, Zhengzhou University and Fudan University), the United States (University of Southern California, The Scripps Research Institute, Arizona State University and the GPCR Consortium), the Netherlands (Vrije Universiteit Amsterdam) and Denmark (Novo Nordisk), provided a detailed molecular map of the full-length human glucagon receptor (GCGR) in complex with a negative allosteric modulator (NNC0640) and the antigen-binding fragment of an inhibitory antibody (mAb1). This study is published together in Nature with a companion paper led by colleagues at the iHuman Institute, Shanghai Tech University describing the glucagon-like peptide-1 receptor (GLP-1R).
Class B GPCRs are essential to numerous physiological processes and serve as important drug targets for many human diseases such as type 2 diabetes, metabolic syndrome, osteoporosis, migraine, depression and anxiety. According to team leader and SIMM professor Dr. Wu Beili, “The GCGR structure provides a clear picture of a full-length class B GPCR at high resolution, and helps us understand how different domains cooperate in modulating the receptor function at the molecular level.” Class B GPCR receptors consist of an extracellular domain (ECD) and a transmembrane domain (TMD), both of which are required to interact with their cognate peptide ligands and to regulate downstream signal transduction. Due to difficulties in high-quality protein preparation, structures of full-length class B GPCRs remained elusive, thus limiting a comprehensive understanding of molecular mechanisms of receptor action.
This study gives some valuable insights into the structure of GCGR. The most exciting finding is that the linker region connecting the ECD and TMD of the receptor, termed the “stalk”, works together with an extracellular loop of the TMD to regulate peptide binding through conformational changes, serving like a modulator in receptor activation. “Although the stalk region only contains 12 amino acids, it acts as a ‘switch’ to turn on or turn off the receptor,” said Dr. Wu. “It is amazing to observe how a GPCR regulates its function in such a precise and efficient way.”
Based on the full-length GCGR structure, the researchers performed a series of functional studies using hydrogen-deuterium exchange, disulfide cross-linking, competitive ligand binding and cell signaling assays as well as molecular dynamics simulations. The results support the GCGR structure and confirm the interactions between different domains in modulating its functionality via conformational alterations. “This study was carried out in a team effort with experts from different fields and different countries. International collaboration is of paramount importance in solving major problems in science nowadays,” said Dr. Jiang Hualiang, director general of SIMM.
“The full-length GCGR structure not only expands our knowledge about GPCR signaling mechanisms, but also offers new opportunities in drug discovery targeting class B GPCRs,” said Dr. Wang Mingwei, Director of the National Center for Drug Screening. “With the information gained from this structure, we are in a better position to devise new therapeutic strategies involving both GCGR and glucagon-like peptide-1 receptor for obesity and type 2 diabetes.”
In addition to Drs. Wu, Wang and Jiang, other study investigators included Dr. Zhao Qiang, Dr. Yang Dehua and two graduate students (Zhang Haonan and Qiao Anna) from SIMM, Dr. Yang Linlin of Zhengzhou University and Dr. Raymond Stevens from the iHuman Institute, Shanghai Tech University. The study was funded by the National Basic Research Programs, the National Health and Family Planning Commission, the National Natural Science Foundation, the Chinese Academy of Sciences, the Shanghai Science and Technology Development Fund, the GPCR Consortium and National Institutes of Health (U.S.A.).
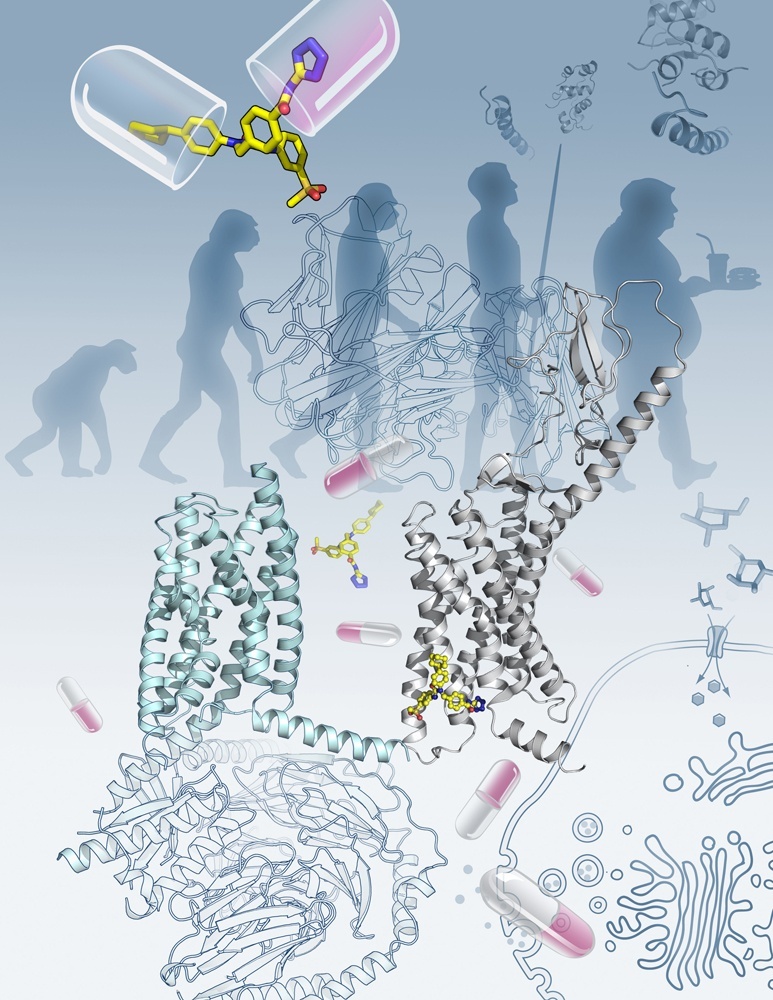
Figure: The new Nature study reports the crystal structure of the full-length human glucagon receptor (GCGR) that plays a key role in glucose homeostasis and serves as an important drug target for type 2 diabetes. The image shows the overall architecture of GCGR (grey cartoon, on the right), which consists of an extracellular domain and a transmembrane domain, in complex with an antibody mAb1 and a negative allosteric modulator NNC0640 (yellow sticks). The recently published cryo-electron microscopy structure of calcitonin receptor (cyan cartoon, on the left) bound to G protein, together with the full-length GCGR structure, highlight the recent breakthroughs in class B GPCR research. (Image by Yekaterina Kadyshevskaya of the Bridge Institute at the University of Southern California)
For more information, please contact:
Prof. Wu Beili
Shanghai Institute of Materia Medica
E-mail address: beiliwu@simm.ac.cn