

Due to the unique properties of fluorine atom(s), fluorinated compounds play important roles in agrochemicals, pharmaceuticals, and materials science. Over the past decade, impressive achievements have been made in efficient introduction of fluorinated groups into organic molecules. However, most of these approaches used well-known and expensive fluorinating reagents; the use of inexpensive, abundant and widely available industrial raw material fluoroalkanes has received less attention because of their relatively inert reactivities. To date, the transformation of these inexpensive fluorine sources remains challenging. Most recently, the Zhang Xingang group from the Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, reported the first example of direct formation of difluoromethylatedarenes from inexpensive and widely available industrial raw material ClCF2H (Nature Chemistry, 2017, DOI: 10.1038/NCHEM.2746; http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.2746.html)(Figure 1 b-c).
Dr. Feng Zhang, Min Qiaoqiao and Fu Xiaping are the co-authors of this paper.
CF2H is not only considered a bioisostere of a hydroxy and thiol group, but also functions as a lipophilic hydrogen bond donor. The selective introduction of CF2H onto aromatic rings can significantly improve their bioactivities compared with their non-fluorinated counterparts. Thus, difluoromethylation is a useful strategy for the modification of biologically active compounds. However, the difluoromethylating reagents used in previous work are expensive and require multistep synthesis. ClCF2H(R22) is the most inexpensive and abundant industrial raw material (1 $/kg) used for the production of various fluorinated polymers (i.e. polytetrafluoroethylene, PTFE) (Figure 1a). From the point of view of cost-efficiency and step-economy, ClCF2H is an ideal and straightforward difluoromethylating reagent; however, the use of ClCF2H for difluoromethylation of aromatics remains a challenge and has not been reported.
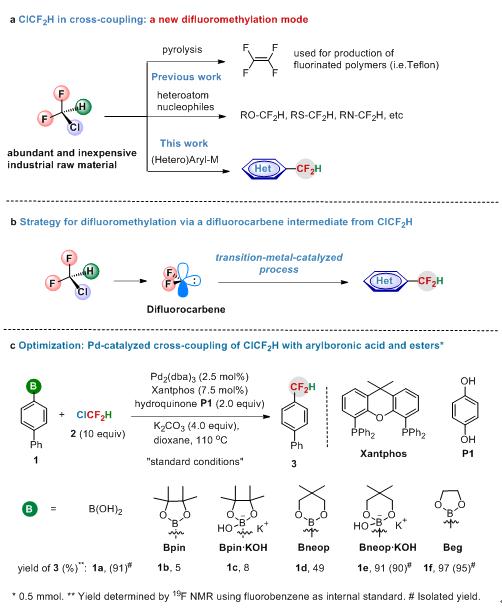
Figure 1.Activation of ClCF2H in organic synthesis
On the basis of their previous transition-metal-catalyzed fluorinations (J. Am. Chem. Soc.2010, 132, 4506; Angew. Chem. Int. Ed.2014, 53, 1669), Zhang’s group has discovered the first example of Pd-catalyzeddifluoromethylation of arylboronic acids with bromodifluoroacetate via a difluorocarbene intermediate (Org. Lett. 2016, 18, 44). Although several difluorocarbene metal complexes have been isolated, the use of metal difluorocarbene to catalyze the reaction remains a great challenge. Inspired by their palladium-difluorocarbene species catalyzed reaction, they have developed the first example of palladium-catalyzed difluoromethylation of (hetero) arylborons with ClCF2H through a transition-metal difluorocarbene pathway, which provides a new mode for activation of ClCF2H (Figure 1b-c). The reaction features several advantages: 1) high efficiency with remarkably broad substrate scope; 2) low-cost difluoromethylating reagent; 3) excellent functional group tolerance, even towards heteroaromatics and biologically active molecular complexes. Most importantly, applications of this cross-coupling can directly introduce a difluoromethyl group at metabolic positions of pharmaceuticals. Even with gram-scale synthesis, good yields were still obtained. Thus, this convenient approach provides a useful tool for the modulation of drugs and biologically active molecules (Figure2). Current efforts are devoted to elucidating the detailed mechanism and lowering the loading amount of catalyst and ClCF2H.
Figure 2. Late-stage difluoromethylation of biologically active molecules
For more information, please contact: Zhang Xingang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, CAS
E-mail: xgzhang@sioc.ac.cn
Source: Shanghai Institute of Organic Chemistry