

Recently, the finite and rising price of fossil resources, climate change from CO2 emission, and other environmental problems have raised interests in polymers from biorenewable raw materials which have a wide variety of biomass resources with low price and enhanced environmental benefits.
Epoxy resins, as one of the three most important thermosetting polymers, have been widely employed in a multitude of fields such as coatings, adhesives, laminated circuit board, electronic component encapsulations, and advanced composites because of their excellent adhesion, chemical resistance, mechanical properties, and dielectric properties.
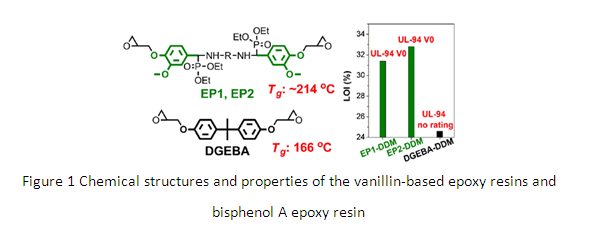

Nowadays, almost all of the epoxy resins are produced from fossil resources, and 90% of the commercially available epoxy resins are diglycidyl ether of bisphenol A (DGEBA) via the reaction of bisphenol A with epichlorohydrin. Bisphenol A, fully dependent on fossil resources, accounts for greater than 67% of the molar mass of DGEBA. In addition, bisphenol A is a reprotoxic compound; as a result, it is under close monitoring, and its application has been restricted in many countries.
The second drawback of epoxy resins is their flammability, which blocks their application in the fire resistance-required fields. Halogen-containing compounds have been regarded as effective agents to improve the flame retardancy of polymeric materials including epoxy resins. With the ban of some halogenated flame retardants such as polybrominated biphenyl (PBB) and polybrominated diphenyl ether (PBDE) by the European Union, the development of an applicable green or halogen-free flame retardant has gained much more attention.
Thus, researchers from the bio-based polymeric materials research group in the Ningbo Institute of Materials Technology & Engineering (CAS) developed two high-performance flame retardant epoxy resins from lignin derivative vanillin and green phosphorus compound diethyl phosphite. Lignin is the second most abundant natural organic material accounting for approximately 30% of organic carbon in the biosphere, and it is also the only scalable renewable feedstock consisted of aromatic monomers, and it is highly underutilized. The two epoxy resins were synthesized by one-pot reaction containing Schiff base formation and phosphorus-hydrogen addition between vanillin, diamines, and diethyl phosphite, followed by reacting with epichlorohydrin, which is relatively green compared with the synthetic routes of the reported vanillin-based epoxy resins using toxic compounds such as oxidant and reducer. Their curing reactivities are similar to bisphenol A epoxy resin DGEBA. After curing they showed excellent flame retardancy with UL-94 V0 rating and high LOI of ~32.8%, which was due to the outstanding intumescent and dense char formation ability. More interestingly, there was no black smoke during the burning test for these two epoxy resins, but a lot of black smoke formed during the burning test for DGEBA. Meanwhile, it was found that the cured vanillin-based epoxies had exceedingly high Tgs of ~214°C, tensile strength of ~80.3MPa, and tensile modulus of ~2709MPa, much higher than the cured DGEBA with Tg of 166°C, tensile strength of 76.4MPa, and tensile modulus of 1893MPa; and the properties of vanillin-based epoxies are easily regulated by using different “coupling” agents -- diamines -- during the synthesis process.
The above-mentioned results have been published in the polymer science journal Macromolecules (2017, 50 (5): 1892–1901).
For more information, please contact:
Dr. Ma Songqi: masongqi@nimte.ac.cn
Prof. Zhu Jin: jzhu@nimte.ac.cn
Source: Ningbo Institute of Industrial Technology, Chinese Academy of Sciences