

Recently, a research group headed by Prof. Qin Yong from the state key laboratory of coal conversion, Institute of Coal Chemistry, Chinese Academy of Sciences(CAS), reported on the design and preparation of a new tandem catalyst with multiple metal-oxide interfaces based on a tube-in-tube nanostructure by applying template-assisted atomic layer deposition (ALD). As an example, they have synthesized a catalyst with both Ni/Al2O3 and Pt/TiO2 interfaces (Al/Ni-Pt/Ti), which exhibits remarkably high catalytic efficiency in the probe tandem reaction of nitrobenzene hydrogenation with hydrogen formed in situ by the decomposition of N2H4·H2O. This can be ascribed to the synergy effect of the two interfaces and the confined nanospace favoring the instant transfer of intermediates. This work has been published in Angew. Chem. Int. Ed. (DOI: 10.1002/ange.201600799;http://onlinelibrary.wiley.com/wol1/doi/10.1002/anie.201600799/abstract),and evaluated as a Very Important Paper (VIP, top 5%) by the reviewers.
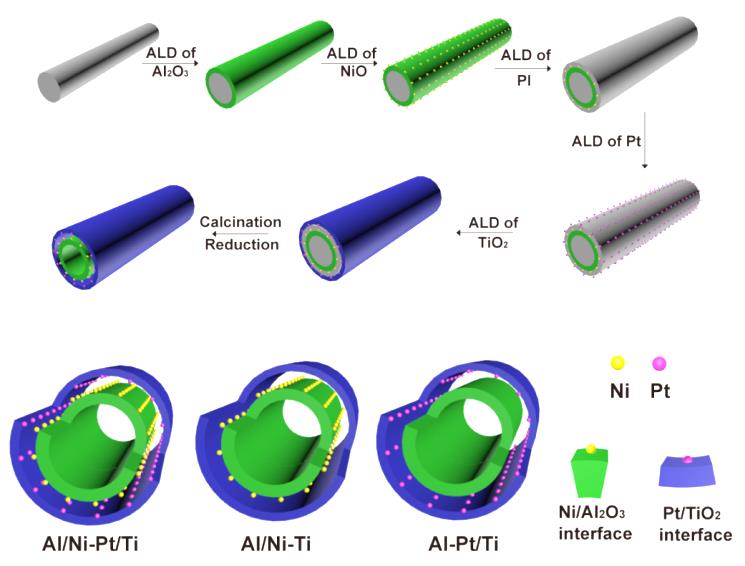
Tandem catalysis has attracted increasing attention, because it can combine multiple chemical transformations in one synthetic operation without separation, purification and transfer of intermediates in each step, thereby saving cost and reducing waste. Metal nanoparticles supported on oxides are widely used in heterogeneous catalysis. The catalytic performance of the metal nanoparticle can be modulated by changing its constitution, shape, size, crystal surfaces, metal-oxide interface, etc. The integration of different metal-oxide interfaces can yield new tandem catalysts for multistep reactions. However, it is difficult to control the composition and microstructure of multiple metal-oxide interfaces at the atomic level by traditional methods.
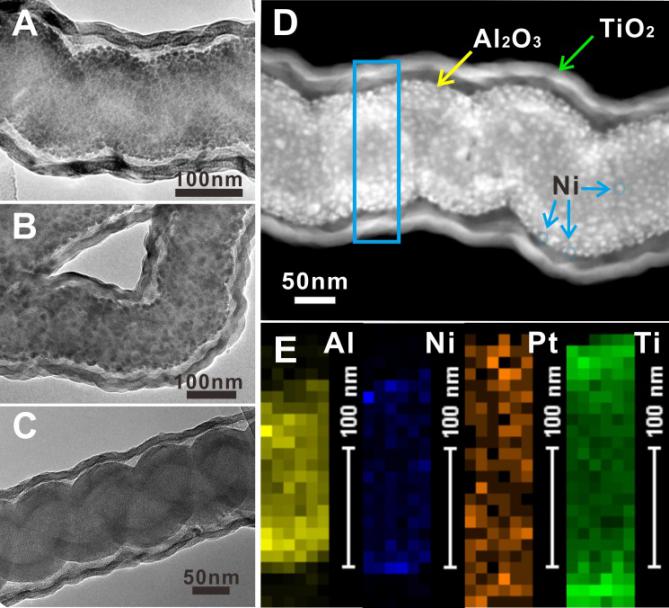
ALD is a high-level film deposition technology, by which metals, oxides, polymers, and other materials are deposited on the surface of substrates via sequential self-limiting reactions. The research group has realized the synthesis of a new tandem catalyst with multiple metal-oxide interfaces via the following route. First, an Al2O3 layer is deposited on the surface of carbon nanocoils (CNCs). Second, NiO nanoparticles are deposited onto the surface of the Al2O3 layer to form NiO/Al2O3 interface. Third, the NiO/Al2O3 interface is coated with polyimide (PI) film to form a sacrificial layer, which is used to control the size of the confined space. Fourth, Pt nanoparticles are deposited on the polyimide (PI) film. Finally, a TiO2 layer is deposited to form the Pt/TiO2 interface. The Al/Ni-Pt/Ti tandem catalyst with Ni/Al2O3 and Pt/TiO2 interfaces is obtained after O2 calcination to remove the template and sacrificial layer and H2/Ar reduction. The catalytic activity of the tandem catalyst is much higher than those of physical mixture catalysts (Al/Ni-Ti+Al-Pt/Ti, or Ni/Al + Pt/Ti).A series of characterization and control experiments have revealed that the active hydrogen can transfer instantly through the confined nanospace. Therefore, the active hydrogen generated from decomposition of N2H4∙H2O over Ni/Al2O3 interface can directly transfer to Pt/TiO2 interface for nitrobenzene hydrogenation without H2 desorption and transfer steps, which has significantly enhanced the catalytic activity of the tandem catalyst.
This achievement has demonstrated a general route to synthesize tandem catalysts with multiple metal-oxide interfaces by ALD. The tube-in-tube tandem catalyst with multiple metal-oxide interfaces represents a new concept for the design of highly efficient and multifunctional nanocatalysts.
What’s more, the group has also synthesized multiple confined nanocatalysts and confined nanocatalysts with ultrathin coating by ALD. Their catalytic activity can be significantly enhanced by changing the metal-oxide interface structure and the number of the interface sites. The relevant works have been published in Angew. Chem. Int. Ed. 2015, 54, 9006-9010 and Chem. Eur. J., 2016, DOI: 10.1002/chem.201601039 (Cover paper).