

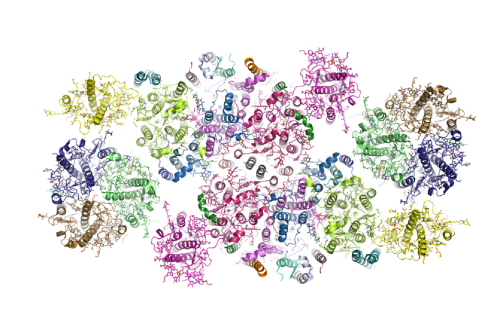
During photosynthesis, the plant photosystem II core complex receives excitation energy from the peripheral light-harvesting complex II (LHCII). The pathways, along which excitation energy is transferred between them, and their assembly mechanisms, remain to be deciphered through high-resolution structural studies. Two research groups headed respectively by Prof. Liu Zhenfeng and Prof. Zhang Xinzheng in close collaboration with the research group headed by Prof. Chang Wenrui and Prof. Li Mei from Institute of Biophysics, Chinese Academy of Sciences (CAS), report the structure of a 1.1-megadalton spinach photosystem II–LHCII supercomplex solved at 3.2 Å resolution through single-particle cryo-electron microscopy. The structure reveals a homodimeric supramolecular system in which each monomer contains 25 protein subunits, 105 chlorophylls, 28 carotenoids and other cofactors. Three extrinsic subunits (PsbO, PsbP and PsbQ), which are essential for optimal oxygen-evolving activity of photosystem II, form a triangular crown that shields the Mn4CaO5-binding domains of CP43 and D1. One major trimeric and two minor monomeric LHCIIs associate with each core-complex monomer, and the antenna–core interactions are reinforced by three small intrinsic subunits (PsbW, PsbH and PsbZ). By analyzing the closely connected interfacial chlorophylls, the researchershave obtained detailed insights into the energy-transfer pathways between the antenna and core complexes.
Powered by solar energy, plants, algae and cyanobacteria convert water and carbon dioxide into organic matter and release oxygen through photosynthesis. In oxygenic photosynthesis, the initial photophysical and photochemical processes are primarily mediated by two photosystems: photosystems I (PSI) and II (PSII). PSII is a supramolecular complex embedded within the thylakoid membrane. It contains numerous protein subunits and various cofactors, including chlorophylls, carotenoids, a Mn4CaO5 cluster, a haem, plastoquinones and lipids. A characteristic functional feature of PSII is its ability to extract electrons from water molecules through a light-induced water-oxidizing reaction catalyzed by the Mn4CaO5 cluster. To collect photon energy and drive the photochemical reactions, plant PSII contains a series of peripheral light-harvesting complexes (the major light-harvesting complexes of PSII (LHCII) and minor ones named chlorophyll-binding protein of 29, 26 and 24 kDa (CP29, CP26 and CP24)). These antenna complexes surround the core complex of PSII, absorb light energy and transmit it to the reaction center to induce charge separation in the special pair of chlorophylls named P680.
The highest resolution at which the crystal structure of cyanobacterial PSII has been solved is 1.9 Å, after a decade-long optimization process starting from 3.8 Å resolution. The structures of cyanobacterial PSII provide solid foundations for understanding the pathways of excitation energy transfer, electron transport and water splitting processes occurring within the complex. Although plant PSII has a core complex similar to that of cyanobacterial PSII, there are major differences in their luminal extrinsic domains and peripheral antenna systems. The structures of partial and intact core complexes of plant PSII have been solved through cryo-electron crystallography at 8–10 Å resolution, and X-ray structures of isolated LHCII and CP29 are available at 2.5–2.8 Å resolution. Furthermore, a 3D map of the PSII–LHCII supercomplex at 17 Å resolution was obtained through single-particle cryo-electron microscopy (cryo-EM), and 2D projection maps of larger supercomplexes with more antenna complexes bound have been reported at 12–13 Å. Nevertheless, the precise pathways of excitation energy transfer between the peripheral antennae and core complex of plant PSII remain largely unclear owing to the lack of a high-resolution structure of the PSII–LHCII supercomplex. Moreover, the structural roles and mutual interactions of three important major extrinsic subunits (PsbO, PsbP and PsbQ) in plant PSII are unknown. The research groups present a 3.2 Å resolution cryo-EM structure of spinach PSII in complex with LHCII, CP29, CP26 and four extrinsic proteins. Unprecedented details concerning the specific interactions between different components within the supercomplex are revealed.
Their achievement was reported online by Nature on May 18 as a thematic article. PhD student Wei Xuepeng and Assistant Research fellow Su Xiaodong are first co-authors of the article.