

Over the past few decades, photodynamic therapy (PDT) has emerged as a promising interventional cancer therapy. The goal of a successful PDT is to evoke a potent and sustained photo-chemical reaction among light, a photosensitizer and tissue oxygen. Upon light irradiation, the photosensitizer converts nontoxic oxygen into cytotoxic reactive oxygen species (ROS) to destroy tumor cells and vasculature. Unfortunately, the clinical application of PDT remains limited in some extent due to potential adverse effects and poor tumor accumulation of photosensitizers. A nanoparticle-based delivery system has been developed as an effective strategy to increase the accumulation efficiency and selectivity of photosensitizer in tumors. During photodynamic reaction, a sufficient oxygen source is highly desirable for a successful PDT. Paradoxically, hypoxia is a hallmark of most solid tumors because of the poor vascular architecture from preexisting venules. Furthermore, the consumption of oxygen during PDT aggravates tumors hypoxia, which potentially dampens the therapeutic effect of oxygen-dependent PDT. To overcome this hypoxia limitation, various strategies have been explored, including combination with other photodynamic mechanisms, or catalyzing intracellular H2O2to O2 .
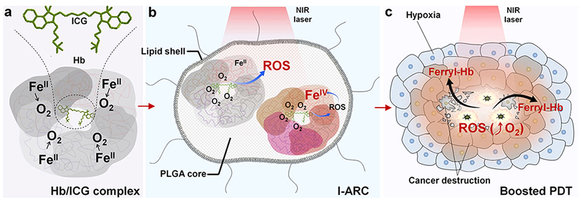
Hemoglobin (Hb) is an iron-rich protein in red blood cells that delivers oxygen to tissues, which could reversibly bind four oxygen molecules to form HbO2. But cell-free Hb is not suitable as an ideal oxygen donor because it produces severe problems, including short circulation time, potential side effects, and poor stability. As a potential new class of oxygen carrier, an Hb-based oxygen carrier via encapsulation with biodegradable materials could successfully conquer the defects of cell-free Hb and obtain an oxygen-carrying capability similar to that of natural red cells. Nano-dimensional oxygen carriers can perfuse tumor tissues within a narrow vascular structure better than red cells, and supply more oxygen to a hypoxic tumor. It is worth noting that iron-rich Hb can be easily oxidized into highly oxidative intermediates so called ferryl-Hb species, resulting in cell injuries by oxidative heme iron.

The researchers of the Nano-medical Group of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (CAS), headed by Professor Cai Lintao, developed a biomimetic lipid-polymer nanoparticle, loading complexes of photosensitizer (indocyanine green, ICG) and oxygen-carrier (Hb), acting as nano-sized artificial red cells to incorporate oxygen supply and monitor the treatment of the photodynamic process. These ICG-loaded artificial red cells (I-ARCs), consisted of a lipid layer of lecithin and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-maleimide (polyethylene glycol 2000) (DSPE-PEG) and a core of poly(lactic-co-glycolic acid) (PLGA) encapsulating Hb/ICG complex. The lipid layer was designed to mimic the cell membrane, and PLGA-encapsulated Hb could reversibly bind four oxygen molecules to form HbO2, with an oxygen carrying function similar to that of red cells. Upon near-infrared (NIR) laser irradiation, ICG could effectively convert nontoxic oxygen to cytotoxic ROS with efficient oxygen supplementation. In the meantime, ROS could oxidize ambient ferrous-Hb to cytotoxic ferryl-Hb species. The outbreak of ROS and far more lasting damage of ferryl-Hb species is eventually triggered to boost destruction to tumors. Moreover, the fluorescence (FL) of ICG and photoacoustic (PA) response of ICG, HbO2 and Hb would dynamically self-monitor the biodistribution and metabolism of components in I-ARCs during PDT.
Photodynamic therapy has been increasingly applied in clinical cancer treatments. However, native hypoxic tumoral microenvironment and a lack of oxygen supply are the major barriers hindering photodynamic reactions. To solve this problem, researchers have developed biomimetic artificial red cells by loading complexes of oxygen-carrier (hemoglobin) and photosensitizer (indocyanine green) for boosted photodynamic effect. Such a nanosystem provides a coupling structure with a stable self-oxygen supply and acts as an ideal fluorescent/photoacoustic imaging probe, dynamically monitoring the nanoparticle biodistribution and PDT. Upon exposure to near-infrared lasers, the remote-triggered photosensitizer generates massive cytotoxic reactive oxygen species (ROS) with sufficient oxygen supply. Importantly, hemoglobin is simultaneously oxidized into the more active and resident ferryl-hemoglobin leading to persistent cytotoxicity. ROS and ferryl-hemoglobin synergistically trigger the oxidative damage to xenograft tumors resulting in complete suppression. The artificial red cells with self-monitoring and boosted photodynamic efficacy could serve as a versatile theranostic platform.
When ICG was introduced to clinics, it was reported that more than 98 percent of ICG bound to proteins (lipoproteins, albumin, etc.) in blood, leading to rapid aggregation and clearance from the body. The researchers then investigated binding properties and molecular interactions between ICG and Hb by computational chemistry using the Internal Coordinate Mechanics (ICM) program (Molsoft). The result revealed that, Hb was made of 4 polypeptide subunits, and ICG occupied the interface close to subunit A and B of the Hb, and the distances from ICG to the four hemes were 17.3, 18.4, 25.4 and 21.3 Å respectively. Each ICG was surrounded by 10 amino-acid residues with extensive electrostatic and hydrophobic interactions, which formed shell interaction with ICG. Consistent with previous report (in electrophoresis, ICG stayed in bands of plasma protein), the result of native-PAGE electrophoresis and FL detection revealed the co-localization of ICG and Hb, which evidenced the formation of ICG/Hb complexes. The Hb/ICG complexes significantly enhanced the encapsulation efficiency to over 95 percent of both Hb and ICG, compared to Hb (86 percent) or ICG (76 percent) alone. Moreover, the proximal distance between ICG and hemes facilitated not only rapid oxygen transport to ICG in photodynamic process, but also simultaneously effective Hb oxidization by ROS, which provided a fundamental basis for the enhanced PDT.
These results were published online in Scientific Reports(Scientific Reports, 2016, 6, 23393) by the Nature Publishing Group as a paper titled “Self-Monitoring Artificial Red Cells with Sufficient Oxygen Supply for Enhanced Photodynamic Therapy” .Luo Zhenyu is the first author of the paper.