

Fischer-Tropsch synthesis (FTS) is a well-known process in industry to convert synthesis gas (a mixture of carbon monoxide, CO and H2) into hydrocarbons. It was invented over 90 years ago and is still in industrial practice today for producing synthetic lubricants and synthetic fuels.
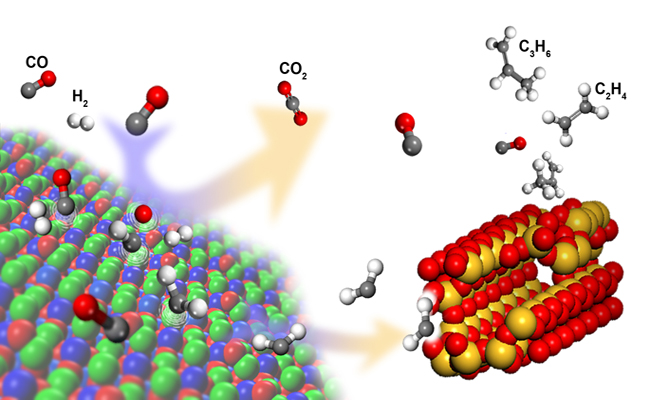
The research team at the Dalian Institute of Chemical Physics (DICP), led by Prof. Bao Xinhe, member of the Chinese Academy of Sciences (CAS), and Prof. Pan Xiulian developed a nanocomposite, which can directly catalyze selective conversion of synthesis gas to light olefins.
The composite catalyst affords two types of active sites with complementary properties. The partially reduced oxide surface activates CO and H2, and C-C coupling is subsequently manipulated within the confined acidic pores of zeolites. Thus, the surface polymerization of CHx is circumvented. This leads to a high selectivity of light olefins (80 percent) and light hydrocarbons C2-C4 (94 percent including olefins and paraffins) at a CO conversion of 17 percent. The C2-C4 selectivity is far beyond the maximum predicted by the Anderson-Schulz-Flory (ASF) model in FTS.
FTS process involves CO and H2 activation over metal surfaces, forming CHx monomers. These monomers go through surface polymerization leading to formation of a wide range of hydrocarbons with different chain lengths. As a result, the selectivity to C2-C4 hydrocarbons, including paraffins and olefins containing two to four carbon atoms, cannot exceed 58 percent.
Furthermore, in the new process, the surface oxygen from dissociated CO is removed by reacting with CO, forming CO2 instead of H2 forming H2O. Thus the energy-intensive water-gas-shift process may be discarded to regulate the ratio of H2/CO. It may allow use of coal- and biomass-derived syngas with a low H2/CO ratio, which could reduce both water and energy-consumption. These findings may open up a new avenue for development of not only syngas-to-olefin technology but also other processes.
This work was published in Science 351: 1065-1068(2016). Prof. Krijn P. de Jong from Utrecht University, a world leading scientist in the field of C1 chemistry, commented in the Perspective, published in the same issue of Science that the research reported by Jiao et al. should be of interest to both academia and industry and that the new process could become a serious competitor for industrial processes such as Fischer-Tropsch to olefins (FTO) and Methanol to olefins (MTO).