

Autism spectrum disorder is a group of neurodevelopment disorders. Patients usually exhibit defects in social interaction, stereotyped repetitive behaviors, anxiety and emotional difficulties. The treatment of autism and the establishment of autism animal models are critical goals in both medicine and neuroscience.
The methylation CpG island binding protein 2 (MECP2) gene has attracted the attention of researchers because of its unique properties among many autism associated genes. Previous work showed that Rett syndrome, a severe neurodevelopment disorder in girls, was caused by mutations of MECP2. On the other hand, duplications of the MECP2 gene led to the MECP2 duplication syndrome, associated with severe autistic behaviors. In animal models, although MECP2-null mice recapitulate most developmental and behavioral defects seen in patients with Rett syndrome, it has been difficult to identify autism-like behaviors in the mouse model of MECP2 over expression.
In order to generate autism models in monkeys, Dr. Qiu Zilong’s Lab and Dr. Sun Qiang’s group at the Institute of Neuroscience (ION), Shanghai Institutes for Biological Sciences (SIBS), Chinese Academy of Sciences (CAS), in collaboration with other relevant research units, constructed a neural specific MECP2 over expressing transgenic monkey by lentiviral infection. Human MECP2 transgenes were effectively inserted into the genome of transgenic monkeys, as confirmed by target sequence capture and deep sequencing analysis. Transgenic monkeys exhibited autism-like behavior, and germ-line transmission of exogenous transgene to offspring was successful, as assayed by genetic and behavior tests. Western blot and immunohistochemical experiments showed that the human MECP2 transgene was properly expressed in the central nervous system.
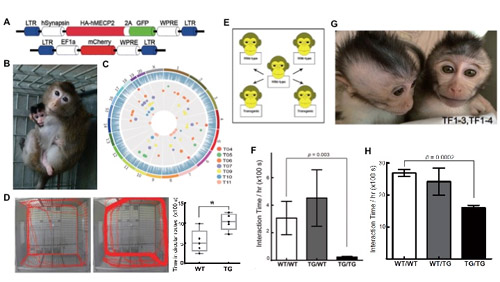
In further study of behavioral phenotypes of MECP2 transgenic monkeys, basic development measurements and an array of behavioral tests were carried out. Comparing with wild monkeys, MECP2 transgenic monkeys gained weight more slowly, had fatty acid metabolism abnormalities, exhibited a higher frequency of repetitive circular locomotion and showed increased stress responses in threat-related anxiety tests. Most importantly, the transgenic monkeys showed less social interaction time with wild monkeys, and also a reduced interaction time when paired with other transgenic monkeys in social interaction tests. The cognitive functions of the transgenic monkeys were largely normal in the Wisconsin general test, although some showed signs of stereotypic cognitive behaviors. These phenotypes were very similar to patients with MECP2 duplication syndrome.
F1 offspring of MECP2 transgenic monkeys were successfully generated by intracytoplasmic sperm injection with sperm from one F0 transgenic monkey, showing germline transmission and Mendelian segregation of MECP2 transgenes in the F1 progeny. Moreover, F1 transgenic monkeys also showed reduced social interactions in the pairing social test, similar to their parental monkeys.
In this project, Qiu’s Lab and the ION Suzhou Facility successfully established a MECP2 transgenic monkey model. The MECP2 transgenic monkeys exhibited autism-like phenotypes, including anxiety responses and defects in social behaviors. F1 transgenic monkeys were successfully generated by intracytoplasmic sperm injection, and also showed autism-like behaviors. As a whole, this work demonstrates the feasibility and reliability of using genetically engineered non-human primates to study brain disorders.
This achievement, entitled “Autism-like behaviors and germ-line transmission in transgenic monkeys overexpressing MECP2”, was published online in Nature on January 26, 2016. Liu Zhen and Li Xiao are the first authors with equal contribution.
For more information, please contact:
Institute of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai 200031, China.
E-mail: zqiu@@ion.ac.cn