

Filoviruses, including Ebola and Marburg, cause fatal hemorrhagic fever in humans and primates. Understanding how these viruses enter host cells could help to develop effective therapeutics.
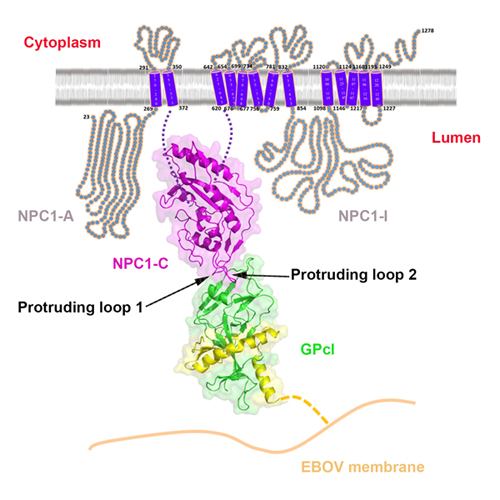
Professor George Fu Gao and his team from the Institute of Microbiology, Chinese Academy of Sciences (CAS) and Chinese Center for Disease Control and Prevention elaborated a new viral membrane fusion mechanism (the fifth mechanism) on the molecular level, which is very different from four kinds of currently well-known viral membrane fusion mechanisms by virologists. This new elaboration is considered as a major breakthrough in the field of virology. The main topics in their study are: the structural basis of Ebola virus endosomal-receptor binding; NPC1 domain C (NPC1-C) displays a helical core structure with two protruding loops; NPC1-C binds to the primed Ebola virus GP (GPcl) protein with a low affinity; and NPC1-C utilizes two protruding loops to engage a hydrophobic cavity on the head of GPcl. An endosomal protein, Niemann-Pick C1 (NPC1), has been identified as a necessary entry receptor for this process, and priming of the viral glycoprotein (GP) to a fusion-competent state is a prerequisite for NPC1 binding. Here, we have determined the crystal structure of the primed GP (GPcl) of Ebola virus bound to domain C of NPC1 (NPC1-C) at a resolution of 2.3 Å. NPC1-C utilizes two protruding loops to engage a hydrophobic cavity on the head of GPcl. Upon enzymatic cleavage and NPC1-C binding, conformational change in the GPcl further affects the state of the internal fusion loop, triggering membrane fusion. Their data therefore provide structural insights into filovirus entry in the late endosome and into the molecular basis for design of therapeutic inhibitors of viral entry.
Their study has provided a new targeted point for the design of anti-virus medicine. Wang Han, Shi Yi, Song Jian, and Qi Jianxun are the first authors, and George Fu Gao is a corresponding author. Their study has greatly deepened the understanding of the virus invasion mechanism, and provided a substantial basis for the control and prevention of Ebola virus. Online publication of their study by the international magazine Cell is entitled Ebola Viral Glycoprotein Bound to Its Endosomal Receptor Niemann-Pick C1, dated January 15, 2016.