

Rechargeable lithium ion batteries (LIBs), one of the most important energy storage technologies, have become an indispensable part of our daily lives. However, safety issues are one of the most urgent concerns associated with further advances in next-generation high-energy batteries. Safety issues such as poor thermal stability, flammable reaction products, leakage of electrolyte and internal short circuits for the use of organic electrolytes in lithium ion rechargeable batteries remain unresolved. The use of a solid electrolyte (SE, based on a polymer or a ceramic) instead of organic electrolytes has been suggested as a preferred solution to these safety issues. All-solid-state lithium batteries employing SEs possess high energy density, no-leakage of electrolytes, flame resistance and flexible geometry. Generally, as a SE for all-solid-state lithium batteries, it needs to maintain electrical isolation between electrodes of opposite polarity while allowing free ionic transport. Therefore, the physical and chemical properties of the electrolyte directly determine the performance of all-solid-state lithium batteries.
Recently, Prof. Xu Xiaoxiong and his team at Ningbo Institute of Materials Technology and Engineering (NIMTE, Chinese Academy of Sciences) have successfully developed a series of novel SE materials. A novel sulfide electrolyte 70Li2S·29P2S5·1Li3PO4 (mol%) glass-ceramic was prepared by a high-energy ball milling technique and subsequent heat-treatment process. The Li+ conductivity is enhanced by the substitution of Li3PO4 for P2S5, and the 70Li2S·29P2S5· 1Li3PO4 glass-ceramics exhibit the highest total conductivity of 1.87×10-3 S cm-1 at 25℃and the lowest activation energy of 18 kJ ·mol-1. The all-solid-state cell In-Li/70Li2S·29P2S5·1Li3PO4 /LiCoO2 exhibits a discharge capacity of 108 mAh·g-1, which is 20% higher than that of the In-Li/70Li2S·30P2S5 /LiCoO2 cell. The higher capacity of a cell assembled by 70Li2S·29P2S5· 1Li3PO4 is attributed to the high Li+ conductivity and low interface resistance of electrode-electrolyte.
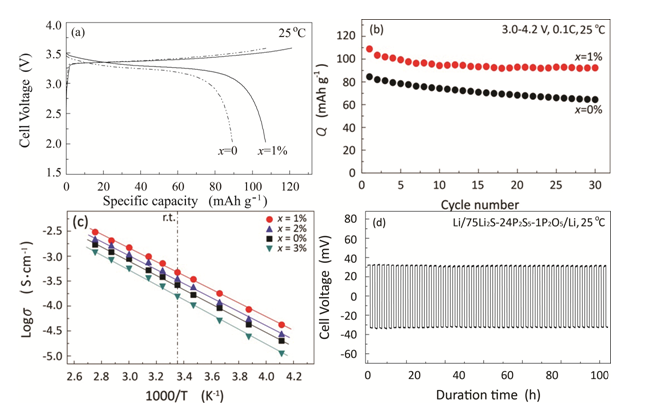
For a high-energy battery using Li metal as a negative electrode, the electrolyte is one of the most critical factors that significantly affect cell performance. Unfortunately, rechargeable batteries based on Li negative electrodes have not been commercialized possibly because of the unstable reactions between Li metal and electrolytes. 75Li2S·25P2S5 (mol%) (Li3PS4) crystal has been reported and demonstrated as one of the most promising electrolyte materials for high-energy batteries with metallic Li negative electrodes, whereas its lithium ionic conductivity is still too low to meet the demand for all-solid-state cells with high capacity. New 75Li2S·(25-x) P2S5·x P2O5 (mol%) solid state electrolytes were synthesized. The electrolyte substituted with 1 mol% P2O5 presents the highest conductivity of 8×10-4 S cm-1at room temperature, which increases up to 56 percent compared to that of the pristine sample. The discharge capacity after 30 cycles of the cell using the 75Li2S·24P2S5·1 P2O5 is 85.2 percent of the initial capacity, whereas that of the cell using the 75Li2S·25P2S5 is 76.2 percent. Besides, the as-prepared 75Li2S·24P2S5·1 P2O5 electrolyte exhibits good electrochemical stability and compatibility against metallic lithium electrodes.
The above results have been published on the Journal of Power Sources (2015) and Journal of the Electrochemical Society(2016). Also, two Chinese patents have been filed (201310535524.6; 201510585679.X).