

Difluoromethylthio group (-SCF2H) is an analog of lipophilic fluoroalkyl group-trifluoromethylthio group (-SCF3). Yet, the properties of SCF2H are quite different from those of SCF3. With a Hansch parameter pR =1.44, SCF3 is one of the most lipophilic groups, while the difluoromethylthio group (Hansch parameter pR = 0.68) is only slightly more lipophilic than the methyl group (pR = 0.56). Such a dramatic change in lipophilicity with a subtle change in structure provides medical chemists an opportunity to fine-tune the drug molecule’s lipophilicity. On the other hand, the difluoromethylthio group, bearing a difluoromethyl group which is generally considered a bioisostere of an OH or NH group, could enhance the drug molecule’s binding selectivity. As a result, development of new efficient methods for the formation of difluoromethylthiolated compounds is of great interest.
One routinely used approach for the preparation of difluoromethylthiolated compounds typically involves the nucleophilic attack of the in situ formed difluoromethyl carbene intermediate by an appropriate thiolate. Nevertheless, these methods were conducted under basic conditions and many functional groups are not compatible. Thus, a new strategy that can form difluoromethylthiolated arenes and heteroarenes under mild conditions is highly desirable.
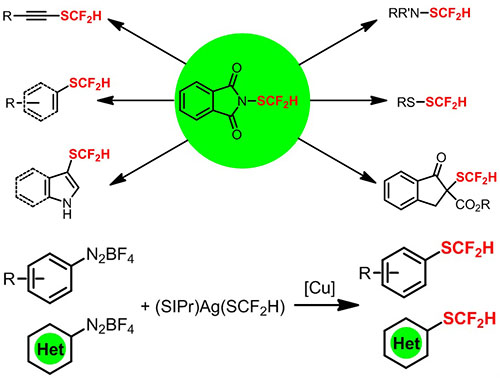
Recently, Shen Qilong’s group at Shanghai Institute of Organic Chemistry developed two reagents including one electrophilic difluoromethylthiolating reagent N-difluoromethylthiophthalimide and one nucleophilic difluoromethylthiolating reagent [(SIPr)Ag(SCF2H)] for direct difluoromethylthiolating a variety of small molecules.
The first electrophilic difluoromethylthiolating reagent N-difluoromethylthiophthalimide can be efficiently synthesized in four steps from the cheap commodity chemical phthalimide. In the presence of Lewis acid Me3SiCl, a copper catalyst, N-difluoromethylthiophthalimide was able to efficiently difluoromethylthiolate a variety of nucleophiles including amines, thiols, β-ketoesters aryl/vinyl boronic acids, alkynes and electro-rich heteroarenes such as oxindoles, indoles, pyrroles 1H-pyrrolo[2,3-b]pyridine, imidazo[1,2-a]pyridine, aminothiazole and isoxazole effectively. The excellent functional group tolerance in these reactions underlines the great potential of N-difluoromethylthiophthalimide as a general reagent for the preparation of more complicated, densely functional drug-like molecules. This work, published recently in J. Am. Chem. Soc. (JACS, 2015, 137, 10547), was highlighted by C&E News (C&E News, 2015, 93(10), 45). Four patents (201510051696.5, 201510051697.X, 201510051924.9, 201510051839.2) about the reagent have been submitted.
The first nucleophilic difluoromethylthiolating reagent [(SIPr)Ag(SCF2H)] 1 could be easily synthesized from the reaction of [(SIPr)Ag(CF2H)], a complex previously discovered in Shen’s laboratory, with sulphur in THF for 50 minutes at ambient temperature. In the presence of a copper catalyst, Sandmeyer type difluoromethylthiolation of aryl and heteroaryl diazonium salts with [(SIPr)Ag(SCF2H)] 1 could be achieved under mild reaction conditions. A variety of functional groups were compatible. The advantage of this method was further demonstrated by application in a number of biologically active molecules. Thus, the current method provides an alternative and attractive strategy for the formation of the difluoromethylthiolated arenes and heteroarenes (Angew. Chem. Int. Ed. 2015, 54, 7648).