

Human aging has become a global issue that brings tremendous challenges and opportunities. As the elderly population suffers more from aging related chronic diseases, which significantly affect their life quality, “Aging Well” has become a global priority and a challenge to the biomedical research field. Lately, a group of scientists led by Professor Liu Guanghui of the Institute of Biophysics, Chinese Academy of Sciences (CAS), reported that they had discovered an important new potential driver of human aging, a finding that could have vast implications for human longevity and the treatment of age-related diseases such as diabetes, Alzheimer's disease and cancer.
Werner syndrome, also known as adult progeria, is a rare disease with symptoms that mimic premature aging. Patients with this disease usually go gray in their 20s, develop cataracts and osteoporosis in their 30s, and die before 60. In studying the genetic mutations underlying Werner syndrome using a stem cell based disease model, researchers discovered that the disorganization of the cellular heterochromatin might be the cause of the accelerated aging.
Manifested symptoms of Werner syndrome suggest cellular defects in patients’ mesenchymal stem cells (MSCs), a specialized stem cell type that is the source of fat, cartilage and bone cells. When the team introduced the WRN gene mutation into human embryonic stem (ES) cells and subsequently differentiated them into MSCs, WRN deficient cells appeared to age more rapidly compared to wild type counterparts. Those cells gradually lost the ability to divide and showed profound signs pointing to a stagnant state known as cellular senescence, such as unregulated inflammation signals and shorter-than-normal telomeres.
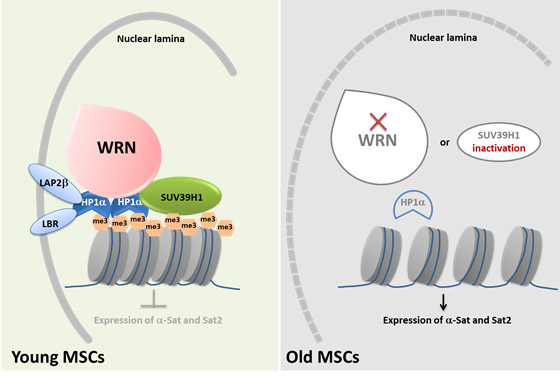
Previously, enormous efforts studying the biological basis of aging had focused on mutations on DNA; however, the new study showed that WRN deficiency in MSCs caused a global loss of H3K9me3 marks and changes in heterochromatin architecture, which are epigenetic alternations well acknowledged with cellular senescence. Further investigation showed that WRN protein associates with heterochromatin proteins SUV39H1 and HP1α and nuclear lamina-heterochromatin anchoring protein LAP2β. Targeted knock-in of catalytically inactive SUV39H1 in wild type MSCs recapitulated accelerated cellular senescence, resembling defects in WRN-deficient MSCs. Even more excitingly, decreases in WRN protein and heterochromatin hallmarks were detected in MSCs samples from old individuals. These new observations uncovered an unprecedented role of WRN in maintaining heterochromatin stability and highlighted heterochromatin disorganization as a potential determinant of human aging. More importantly, though cautious and more extensive studies shall be conducted in the future, the current report suggests a novel possibility to slow down or even reverse human cellular aging by keeping DNA more stably packed together in the cells, which implicates a new hope for anti-aging therapy.
This new discovery has been highlighted by many media reports and commented by influential scientists. Professor Liu Guanghui, a scientist from the Chinese Academy of Sciences who is the senior author of the paper and collaborated with researchers from Peking University and the Salk Institute (California, USA), reported this new finding in Science magazine on April 30, 2015.