

On June 21, 2013, the first human case of infection with avian influenza A (H6N1) virus was reported in Taiwan. Sequence analyses reveal that the human isolate (A/Taiwan/2/2013) from the patient is highly homologous to the chicken H6N1 virus isolates in Taiwan. Researchers led by Professor Gao Fu (George Fu Gao) at the Institute of Microbiology, Chinese Academy of Sciences (CAS), have made progress in understanding the molecular mechanism of cross-species transmission of the H6N1 influenza virus. Their findings were published online in The EMBO Journal on May 4.
Infections with the H6N1 virus have been prevalent in domestic chickens in Taiwan, and the virus circulating in Taiwan poultry has developed into a genetically unique lineage, different from the H6 subtype viruses circulating in Hong Kong and South China. Researchers performed comprehensive analysis of key residues in the receptor-binding site of Taiwan-isolated H6 HAs from 1972 to 2013 and selected representative strains to detect their receptor binding properties from the protein and virus level. They proposed that the evolution of receptor-binding properties of Taiwan-isolated H6 HAs underwent two major processes: initially avian receptor-binding preference and secondarily dual receptor-binding activity. On the other hand, the H6N1 virus that caused human infection had a human receptor-binding preference. Mutagenesis work revealed that amino acids at the position of 186, 190, 228 played an important part in the switch of receptor-binding preference. E190V and G228S substitutions were important to acquire the human receptor-binding capacity, and the P186L substitution could reduce the binding to avian receptor. At the same time, researchers elucidated the structural basis of how the P186L substitution in the receptor-binding site of HA determines the receptor-binding preference change.
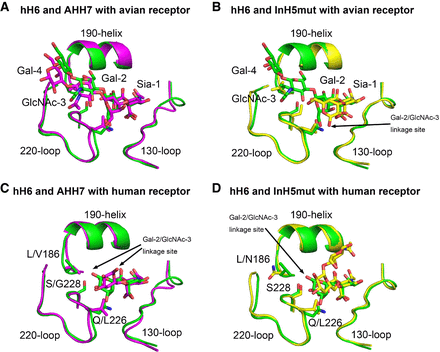
Researchers thought that the affinity for the human receptor is low for most of the H6 subtype viruses. High affinity for the avian receptor has been maintained, which may hamper efficient infection and transmission in humans. But the H6 subtype viruses will continue to evolve, especially gaining internal genes from H9N2 (as happened for the recent H7N9 virus), increasing the potential risk of human infection and even pandemics. The important thing is this human-infecting H6N1 influenza virus has already acquired the human receptor binding preference, which was similar to the human influenza virus. It is very likely to cause pandemics once mutations occur in other genes together with this.
Wang Fei, post-doctoral student of Professor Gao Fu at the College of Veterinary Medicine, China Agricultural University, and Qi Jianxun from the Institute of Microbiology, CAS, are the first authors. This work also received help from Shi Yi (Beijing Institutes of Life Science, CAS), Zhang Wei, Bi Yuhai, Yan Jinghua (Institute of Microbiology, CAS), Wang Ming and Liu Jinhua (College of Veterinary Medicine, China Agricultural University).